Mechanisms of centrosome regulation
Centrosomes are small organelles comprising an inner pair of centrioles surrounded by a proteinaceous matrix that is the pericentriolar material. The composition and organization of the pericentriolar material instructs the microtubule-organizing activity of centrosomes.
In many cell types, the cell cycle-dependent cycling of pericentriolar recruitment and shedding is associated with a dynamic reconfiguration of the centrosome structure and contributes to the diversity of centrosome functions.
We investigate how changes in centrosome activity are regulated in space and time. Many of these changes are critical for normal growth and development. In contrast, impaired centrosome activity control contributes to the pathophysiology of disease. Centrosome dysfunction, for example, results in cell division defects and genome instability associated with cancer and neurodevelopmental disorders.
Coordinated regulation of centrosome activity in stem cells
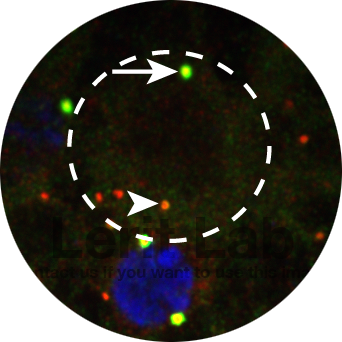
Neural stem cells (dashed circle) show marked asymmetries in centrosome activity control. Active daughter centrosome (arrow) and inactive mother centrosome (arrowhead) are highlighted. Pericentriolar material is labeled in green; centrioles are red.
To permit error-free mitosis, all cells must duplicate their centrosomes once (and only one) prior to mitotic onset. An older centrosome (the mother) serves as a template for the genesis of a new (daughter) centrosome. In many stem cells, individual centrosomes are differentially regulated within the context of a single cell. The neural stem cells of the Drosophila larval brain divide along an invariant apical-to-basal polarity axis that is entrained by centrosome age and position. Division of these stem cells is required for neurogenesis, and defective neural stem cell divisions are associated with neurodevelopmental disorders and brain tumors.
Before mitosis, the mother and daughter centrosomes of neural stem cells display marked asymmetries in centrosome maturation. The daughter centrosome recruits more pericentriolar material than the mother centrosome. We identified a key role for Pericentrin-like protein (PLP), a centrosome scaffolding factor, in regulating the asymmetric maturation of neural stem cell centrosomes (Lerit et al., 2013 JCB). Mutation of the mammalian ortholog, Pericentrin, is associated with microcephaly (small brain), suggesting many of the core functions of PLP and Pericentrin are functionally conserved.
We interrogate the fascinating question of how centrosome asymmetry is established and how such asymmetries contribute to neurodevelopment and tissue homeostasis.
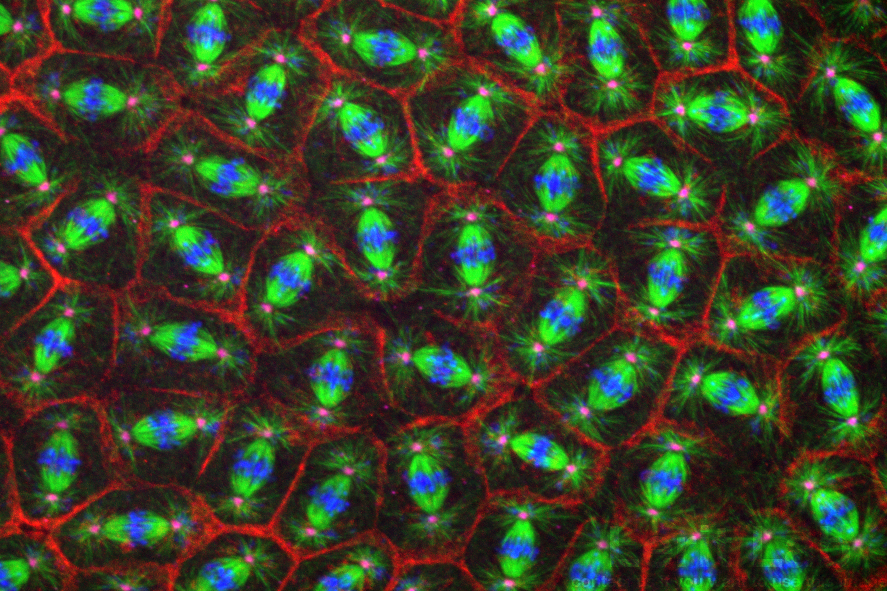
During interphase (left) the daughter centrosome (arrow) is a more “active” microtubule-organizing center than the mother centrosome (arrowhead). During mitosis (right), both centrosomes (arrows) undergo mitotic maturation and contribute to spindle formation. Microtubules are shown in green; centrioles are red; nuclei of mitotic cells are blue.
Dynamic rearrangements of centrosomes during embryogenesis
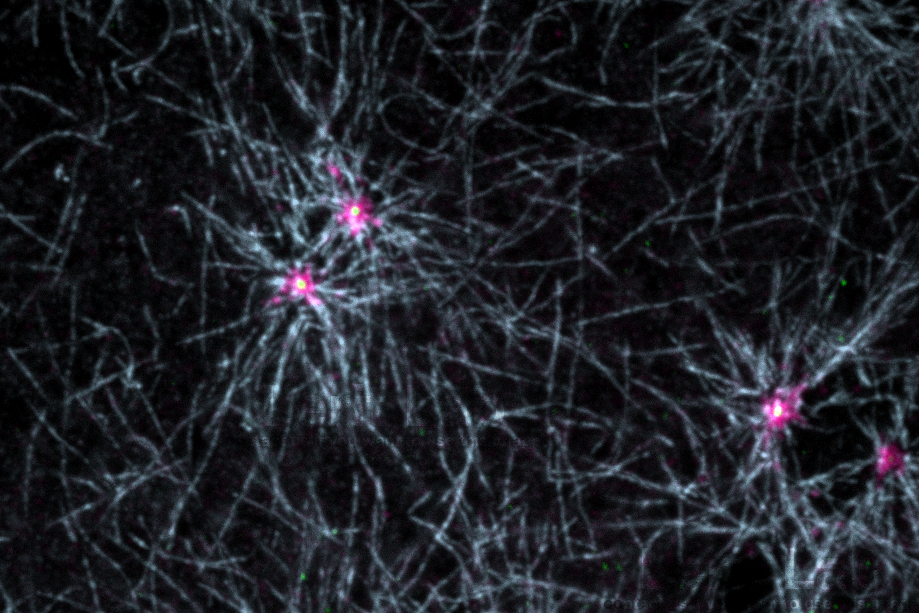
Microtubule nucleation in early embryos. Microtubules (blue) radiate from centrosome flares (pericentriolar material, magenta). Centrioles (yellow) reside within the center.
Pericentriolar material recruitment and shedding dictates the cell cycle-dependent oscillations in centrosome size. Importantly, these variations also contribute to the multifunctional nature of centrosomes. We use the early Drosophila embryo as a model to study the differential regulation of interphase versus mitotic centrosomes. Given the unique developmental properties of this system, embryonic centrosomes are ideal for high-resolution imaging of centrosomes. Remarkably, the vast majority of centrosome genes are conserved from flies to humans.
We identified the coordinated function of PLP and another pericentriolar material constituent, Centrosomin/CDK5RAP2 (Cnn), in organizing an interphase-specific centrosome scaffold (Lerit et al., 2015 JCB). These fibrillar structures, called centrosome flares, serve an important centrosome structural role. Loss of this scaffold perturbs microtubule organization and causes genome instability.
We investigate the differential composition and organization of interphase versus mitotic centrosomes and how these differences contribute to cellular and animal viability. We recently identified mRNA as an entire class of biomolecules whose recruitment to centrosomes is both developmentally and cell cycle-regulated. We are investigating the role of these localized mRNAs, as well as the post-transcriptional regulatory paradigms of core centrosome components.
mRNA (magenta) enrichment at centrosomes (green).
Individual mRNA molecules (red) cluster at the centrosomes (green).
Requirement of centrosomes in germline development
Primordial germ cells (PGC) are progenitors to the adult germline stem cells. We previously demonstrated a requirement for centrosomes to ensure the faithful segregation of the germline fate determinants, or germ plasm, into nascent primordial germ cells as an early event that establishes the soma versus germline dichotomy in Drosophila (Lerit and Gavis, 2011, Current Biology).
In addition to directing the passage of cell fate molecules into PGCs, centrosomes also contribute to the formation of these cells. Our work is consistent with a fundamental role for centrosomes in the formation of PGCs.
We recently discovered the germ plasm factor, Germ cell-less (GCL), influences centrosome dynamics (Lerit et al, 2017 Cell Reports), and we’ve uncovered additional centrosome factors critical to permit germline development (Fang and Lerit, 2020, Genesis). We study the intriguing coordination of centrosome regulation and germ cell development as a model toward understanding how centrosomes instruct cell fate decisions, drive asymmetric cell divisions, and direct the trafficking of fate molecules.
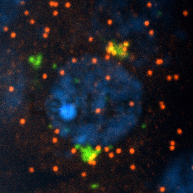
An early step in PGC formation is the recruitment of the germ plasm (green) to centrosomes (red, arrows). Budding posterior membrane, magenta.
Microtubules (green) nucleated by centrosomes (magenta) partition the germ plasm (red) into PGCs.
Funding
We are grateful for funding support provided by the Department of Cell Biology at Emory University School of Medicine, the Robert W. Woodruff Fund, Inc., and the following: